- Home
- INTERMEDIATE ALGEBRA
- Course Syllabus for Algebra I
- Mid-Plains Community College
- FRACTION OF A WHOLE NUMBER
- Systems of Linear Equations
- MATH FIELD DAY
- Course Outline for Finite Mathematics
- Calculus
- Algebra Final Examination
- Math 310 Exam #2
- Review of Trigonometric Functions
- Math 118 Practice test
- Precalculus Review
- Section 12
- Literal Equations
- Calculus Term Definitions
- Math 327A Exercise 2
- Public Key Algorithms II
- Maximizing Triangle Area
- Precalculus I Review for Midterm
- REVIEW OF A FIRST COURSE IN LINEAR ALGEBRA
- Math 6310 Homework 5
- Some Proofs of the Existence of Irrational Numbers
- ALGEBRAIC PROPERTIES OF MATRIX OPERATIONS
- Math 142 - Chapter 2 Lecture Notes
- Math 112 syllabus
- Math 371 Problem Set
- Complex Numbers,Complex Functions and Contour Integrals
- APPLICATIONS OF LINEAR EQUATIONS
- Week 4 Math
- Fractions
- Investigating Liner Equations Using Graphing Calculator
- MATH 23 FINAL EXAM REVIEW
- Algebra 1
- PYTHAGOREAN THEOREM AND DISTANCE FORMULA
- Georgia Performance Standards Framework for Mathematics - Grade 6
- Intermediate Algebra
- Introduction to Fractions
- FACTORINGS OF QUADRATIC FUNCTIONS
- Elementary Algebra Syllabus
- Description of Mathematics
- Integration Review Solutions
- College Algebra - Applications
- A Tip Sheet on GREATEST COMMON FACTOR
- Syllabus for Elementary Algebra
- College Algebra II and Analytic Geometry
- Functions
- BASIC MATHEMATICS
- Quadratic Equations
- Language Arts, Math, Science, Social Studies, Char
- Fractions and Decimals
- ON SOLUTIONS OF LINEAR EQUATIONS
- Math 35 Practice Final
- Solving Equations
- Introduction to Symbolic Computation
- Course Syllabus for Math 935
- Fractions
- Fabulous Fractions
- Archimedean Property and Distribution of Q in R
- Algebra for Calculus
- Math112 Practice Test #2
- College Algebra and Trigonometry
- ALGEBRA 1A TASKS
- Description of Mathematics
- Simplifying Expressions
- Imaginary and Complex Numbers
- Building and Teaching a Math Enhancement
- Math Problems
- Algebra of Matrices Systems of Linear Equations
- Survey of Algebra
- Approximation of irrational numbers
- More about Quadratic Functions
- Long Division
- Algebraic Properties of Matrix Operation
- MATH 101 Intermediate Algebra
- Rational Number Project
- Departmental Syllabus for Finite Mathematics
- WRITTEN HOMEWORK ASSIGNMENT
- Description of Mathematics
- Rationalize Denominators
- Math Proficiency Placement Exam
- linear Equations
- Description of Mathematics & Statistics
- Systems of Linear Equations
- Algebraic Thinking
- Study Sheets - Decimals
- An Overview of Babylonian Mathematics
- Mathematics 115 - College Algebra
- Complex Numbers,Complex Functions and Contour Integrals
- Growing Circles
- Algebra II Course Curriculum
- The Natural Logarithmic Function: Integration
- Rational Expressions
- QUANTITATIVE METHODS
- Basic Facts about Rational Funct
- Statistics
- MAT 1033 FINAL WORKSHOP REVIEW
- Measurements Significant figures
- Pre-Calculus 1
- Compositions and Inverses of Functions
Measurements Significant figures Scientific Notation
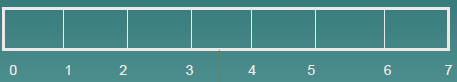
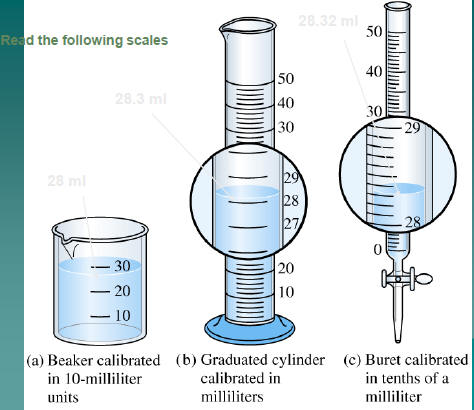
Read the following scales

3 units
Reading
3.4 units
Reading
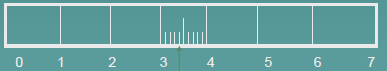
3.41 units
Reading
Uncertainty in
measurement
Read the following
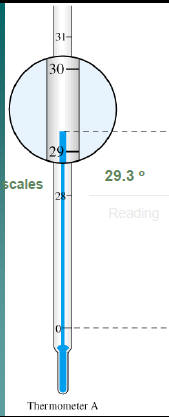
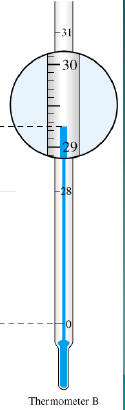
29.25°
Reading
Read the following scales
Uncertainity in a measured number
 To determine the uncertainity in a number
To determine the uncertainity in a number
–look at the last digit –this is the uncertain digit
–402.3 Last digit is 3 and is in the tenths place
 The uncertainity is + 0.1
The uncertainity is + 0.1
–402.34 Last digit is 4 and is in the hundredths place
 The uncertainity is + 0.01
The uncertainity is + 0.01
Uncertainity in a measured number
 Examples: continued
Examples: continued
–230 Last significant digit is 3. 3 is in the tens place
 The uncertainity is + 10
The uncertainity is + 10
 This means our measurement is 230, 220 or 240
This means our measurement is 230, 220 or 240
Errors in Measurement
Random errors are errors originating from
uncontrolled variables in the experiment.
Experimental values that fluctuate about the true value.
Systematic errors are errors originating from
controlled variables in the experiment.
-constant errors
-occur again and again
-affect the accuracy of the measurements can occur due miss-calibration of a
measuring device
-are readings in variables such as temperature, pressure, flow, weight and alike
Driving down the thruway to MCC
| Weight your car to the nearest 1 lb $50.00 |
| Weight your car to the nearest 10 lb $15.00 |
| Weight your car to the nearest 100 lb $2.00 |
Cont.
![]() Weight Limit 2556 and
we mean it!.
Weight Limit 2556 and
we mean it!.
Car manual reads weight to be between 2421 to 2707
depending on # people in car and what’s in the trunk
![]() At
$2.00 station weight is 2600 which
At
$2.00 station weight is 2600 which
means car weight is 2500-2700 lbs
![]() At $15.00 station
weight is 2550
At $15.00 station
weight is 2550
which means car weight is 2540-2560 lbs
![]() At $2.00 station
weight is 2549
At $2.00 station
weight is 2549
which means car weight is 2548-2550 lbs
![]() Will
the bridge collapse?
Will
the bridge collapse?
![]() Discussion
Discussion
Significant Figures
Significant Figures rules
•The digits 1 to 9 inclusive always count as
significant figures
14.23
3.112
244.62
Leading zeros and zeros that occur at the start of
a number , do not count for sig. figs. They only
indicate position
.004
.00036
0.00125
![]() Zeros
between nonzero digits count for sig.fig.
Zeros
between nonzero digits count for sig.fig.
3.075
1005
.030078
![]() Zeros at the end of
the number are only
Zeros at the end of
the number are only
significant if they are after a decimal point
(trailing zeros)
100.030
50.0
.1000
![]() If
you can transform the number to scientific
If
you can transform the number to scientific
notation and the zeros are lost, they were not
significant
![]() 93,000,000 = 9.3 x
10^7
93,000,000 = 9.3 x
10^7
How many significant figures are in
each of the following measurements?
| 24 mL | 2 significant figures |
| 3001 g | 4 significant figures |
| 0.0320 m3 | 3 significant figures |
| 6.4 x 104 molecules | 2 significant fig |
| 560 kg | 2 significant figures |
Significant Figures
Addition or Subtraction
The answer cannot have more digits to the right of the decimal
point than any of the original numbers.
 |
one significant figure after decimal point round off to 90.4 |
 |
two significant figures after decimal point round off to 0.79 |
Problems:
Add 5.62 + 0.0223 + 9.831
Least precise quantity is 5.62 1/100thplace
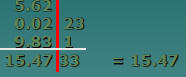
Add 0.02457 + 1.00001 + 0.003
Least precise quantity is 0.003 1/1000thplace

Problems:
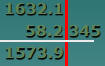
Substract 1632.1 -58.2345
Least precise quantity is 1632.1 1/10thplace
Significant Figures
Multiplication or Division
The number of significant figures in the result is set by the original
number that has the smallest number of significant figures
 |
|
| 3 sig figs | round to 3 sig figs |
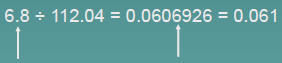 |
|
| 2 sig figs | round to 2 sig figs |
Significant Figures
Exact Numbers
Numbers from definitions or numbers of objects are considered
to have an infinite number of significant figures
The average of three measured lengths; 6.64, 6.68 and 6.70?
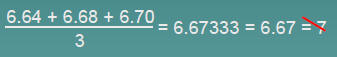
Because 3 is an exact number
Scientific Notation
The number of atoms in 12 g of carbon:
602,200,000,000,000,000,000,000
6.022 x 1023
The mass of a single carbon atom in grams:
0.0000000000000000000000199
1.99 x 10-23
| N x 10n |
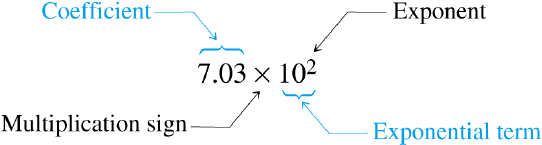
Scientific Notation
consists of two parts.
Scientific notation: Adding exponents
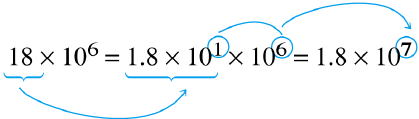
| Express 18 as 1.8 x 101 | Add exponents |
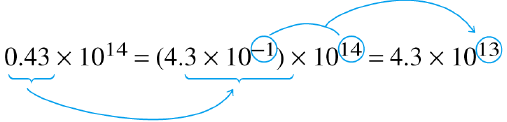
| Express 0.43 as 4.3 x 10-1 | Add exponents |
Scientific Notation
| 568.762 | 0.00000772 |
| ←move decimal left | →move decimal right |
| n > 0 | n < 0 |
| 568.762 = 5.68762 x 102 | 0.00000772 = 7.72 x 10-6 |
| Addition or Subtraction | |
| 1.Write each quantity with the same exponent n | 4.31 x 104+ 3.9 x 103= |
| 2.Combine N1and N2 | 4.31 x 104+ 0.39 x 104= |
| 3.The exponent, n, remains the same | 4.70 x 104 |
Scientific Notation
1.86 x 105would be the
Number.
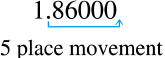
Scientific Notation
3.2 x 1020
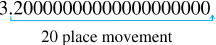
Scientific Notation
1.6 x 10-3would be the number
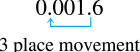
Scientific Notation
1.0 x 10-7would be the number
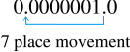
Scientific Notation
Multiplication
| 1.Multiply N1and N2 | 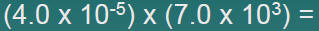 |
| 2.Add exponents n1and n2 | 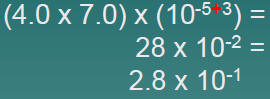 |
| Division | 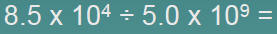 |
| 1.Divide N1and N2 |  |
| 2.Subtract exponents n1and n2 | 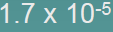 |
Accuracy–how close a measurement is to the true value
Precision–how close a set of measurements are to each other
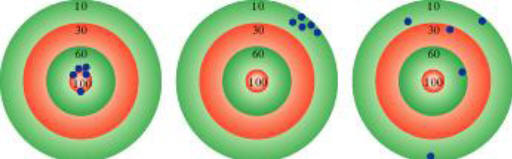 |
||
| accurate & precise |
precise but not accurate |
not accurate & not precise |


